Les facteurs démographiques ont-ils une influence sur l’utilisation de la doravirine?
Review | 2020 Khalilieh S. et al.

Dyslipidaemia is a prevalent cardiovascular risk factor in people living with HIV (PLWH).1 In addition to common contributors of atherosclerosis (such as physiological aging, smoking or diabetes), individual antiretroviral therapy (ART) drugs have been associated with heterogenous cardiometabolic effects in PLWH.2 Regarding nucleoside reverse transcriptase inhibitors (NRTIs), studies in virologically suppressed PLWH showed that switching from tenofovir disoproxil fumarate (TDF) to a tenofovir alafenamide (TAF)-based regime led to weight gain and increased lipid parameters.3,4
In previous studies, changes in lipid parameters after TDF-to-TAF switch were found to be small and their clinical relevance was unclear.5 The aim of the study from Lacey et al.5 was to characterize lipid changes and changes in the proportion with dyslipidaemia after switching to a TAF-containing ART in a real world setting.
In this prospective study, Lacey et al. studied adult PLWH (>18 years) enrolled in the Irish UCD ID Cohort who switched their ART to TAF-containing regimens. Of 775 PLWH in the cohort, 194 patients had switched to TAF-containing ART regimens within the observed study period (January 2016 – July 2017) and had pre- and post-TAF switch lipid profile measurements.
Preswitch lipids were measured a median 16 (0 – 54) weeks prior to switch; postswitch lipids at a median 24 (14 – 41) weeks after switching.
Selected characteristics of the 194 PLWH included in the analysis:
Collated data:
The main outcome was the measurement of lipid parameters postswitch to a TAF-containing regime. A secondary outcome was the postswitch change in the proportion of PLWH with dyslipidaemia, based on the National Cholesterol Education Program-Adult Panel III (NCEP-ATPIII) classification.
Postswitch changes in lipid parameters
Lipid levels significantly increased in PLWH who switched to a TAF-containing ART (Figure 1a). In percentages, there was a postswitch increase in lipid concentrations of:
Similarly, lipid levels significantly increased in a sensitivity analysis of PLWH (n=72) with TDF-to-TAF switch, but no change in other ART drugs. Within this group, there was no significant postswitch change for HDL levels (Figure 1b).

Postswitch changes in NCEP-ATPIII classification
Overall, there was a significant change in NCEP-ATPIII classification (clinical guidelines for cholesterol testing and management, which define threshold values for lipid parameters6) postswitch for TC and LDL, but not for TG and HDL. Comparing pre- to postswitch, there was a significant increase in the proportions of PLWH with abnormal to very high-grade abnormal dyslipidaemia for TC (5.2 vs. 15.5%, p<0.001) and LDL (23.7 vs. 36.6%, p<0.001) (Figure 2).
Changes in NCEP-ATP III classification (i.e. more severe dyslipidaemia postswitch across TC and LDL) were similar for the subgroup analysis of 72 PLWH with TDF-to-TAF switch, but no other changes in ART components.
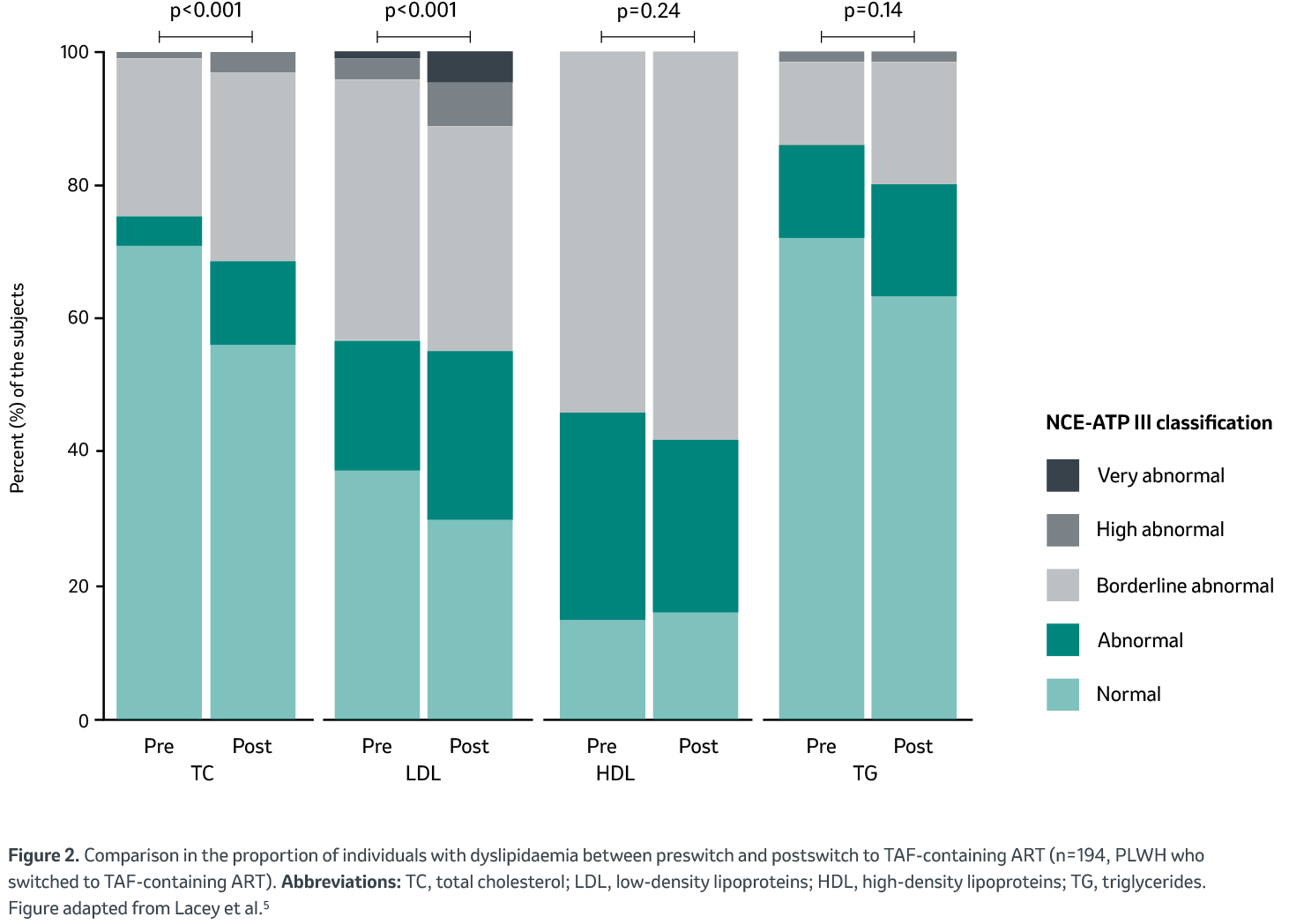
In this study, Lacey et al. described a significant worsening of lipid profiles after switching to TAF from any ART regime. In addition, the authors observed more severe dyslipidaemia postswitch across TC and LDL, which could highlight the clinical relevance of these changes. Similar findings existed in patients who solely switched TDF to TAF while maintaining other antiretroviral components. This could indicate an effect predominantly driven by the TAF-switch, rather than a change in third agents.
However, the authors also referred to the limitations of their study, such as 23.7% of study participants being on lipid lowering therapy prior to the switch, which may have masked the real effect on lipids.
The authors concluded that the development of dyslipidaemia should be considered alongside other parameters such as bone and renal safety when choosing between TDF and TAF.
PLWH:People living with HIV, ART: antiretroviral therapy, NRTI: nucleoside reverse transcriptase inhibitor, TAF: tenofovir alafenamide, TDF:tenofovir disoproxil fumarate, TC:total cholesterol, HDL: high-density lipoprotein, LDL:low-density lipoprotein, TG: trigylcerides, NCEP-ATP III: National Cholesterol Education Program-Adult Panel III.
1. Grand, M., Bia, D. & Diaz, A. Cardiovascular Risk Assessment in People Living With HIV: A Systematic Review and Meta-Analysis of Real-Life Data. Curr. HIV Res. 18, 5–18 (2020). 2. Fragkou, P. C. et al. Cardiovascular disease and risk assessment in people living with HIV: Current practices and novel perspectives. Hell. J. Cardiol. HJC Hell. Kardiologike Epitheorese 71, 42–54 (2023). 3. Mallon, P. W. et al. Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J. Int. AIDS Soc. 24, e25702 (2021). 4. Schafer, J. J. et al. Changes in Body Mass Index and Atherosclerotic Disease Risk Score After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide. Open Forum Infect. Dis. 6, ofz414 (2019). 5. Lacey, A. et al. Investigating the effect of antiretroviral switch to tenofovir alafenamide on lipid profiles in people living with HIV. AIDS Lond. Engl. 34, 1161–1170 (2020). 6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001).
Copies of the study publication can be requested on demand dpoc.switzerland@msd.com.
Before prescribing, please consult the full prescribing information, published on the website of Swissmedic (www.swissmedicinfo.ch).

Review | 2020 Khalilieh S. et al.
Review | 2019 Boyle A. et al.
Vous trouverez ici des nouvelles intéressantes sur les études cliniques dans le domaine du VIH, des mises à jour de guidelines ainsi que des nouvelles sur nos produits.